Ideje Atom Economy Formula Gcse
Ideje Atom Economy Formula Gcse. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.
Tady Moles Calculations Gcse By Polarity24 Teachers Pay Teachers
Ch4 (g) + h2o (g) → co (g) + … 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:
The reaction is as follows: The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.
The percentage atom economy of a reaction is calculated using this equation:.. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The reaction is as follows: The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation:

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. Ch4 (g) + h2o (g) → co (g) + …

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation:

22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: 22/02/2017 · find my revision workbooks here:

The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

Ch4 (g) + h2o (g) → co (g) + …. 22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:. Ch4 (g) + h2o (g) → co (g) + …

22/02/2017 · find my revision workbooks here:.. The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation:. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation:. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

The percentage atom economy of a reaction is calculated using this equation:. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … 22/02/2017 · find my revision workbooks here:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Ch4 (g) + h2o (g) → co (g) + …

22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy... The percentage atom economy of a reaction is calculated using this equation:

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows:.. 22/02/2017 · find my revision workbooks here:

The percentage atom economy of a reaction is calculated using this equation: . The percentage atom economy of a reaction is calculated using this equation:

Ch4 (g) + h2o (g) → co (g) + …. Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here:.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy... Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22/02/2017 · find my revision workbooks here:.. The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation:. The reaction is as follows:

22/02/2017 · find my revision workbooks here: The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. Ch4 (g) + h2o (g) → co (g) + …

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + ….. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation:.. The reaction is as follows:

Ch4 (g) + h2o (g) → co (g) + …. The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The reaction is as follows: 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows:

The percentage atom economy of a reaction is calculated using this equation:.. . Ch4 (g) + h2o (g) → co (g) + …

22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here:.. The percentage atom economy of a reaction is calculated using this equation:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

22/02/2017 · find my revision workbooks here: 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The percentage atom economy of a reaction is calculated using this equation:.. The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:

22/02/2017 · find my revision workbooks here: .. 22/02/2017 · find my revision workbooks here:

Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here: The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation:. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

The reaction is as follows:.. Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The reaction is as follows: 22/02/2017 · find my revision workbooks here:.. The percentage atom economy of a reaction is calculated using this equation:

22/02/2017 · find my revision workbooks here:. 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + …

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows:.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Ch4 (g) + h2o (g) → co (g) + …. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation:. The reaction is as follows:

22/02/2017 · find my revision workbooks here: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows:. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + …. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.
22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation:. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Ch4 (g) + h2o (g) → co (g) + …

The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation:. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage atom economy of a reaction is calculated using this equation:. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch4 (g) + h2o (g) → co (g) + …. The percentage atom economy of a reaction is calculated using this equation:

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows:.. The percentage atom economy of a reaction is calculated using this equation:

22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + ….. Ch4 (g) + h2o (g) → co (g) + …

The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. The reaction is as follows:

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here:.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. . Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22/02/2017 · find my revision workbooks here: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation:. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation:

The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

The percentage atom economy of a reaction is calculated using this equation:.. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows:

The reaction is as follows:. The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22/02/2017 · find my revision workbooks here:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.
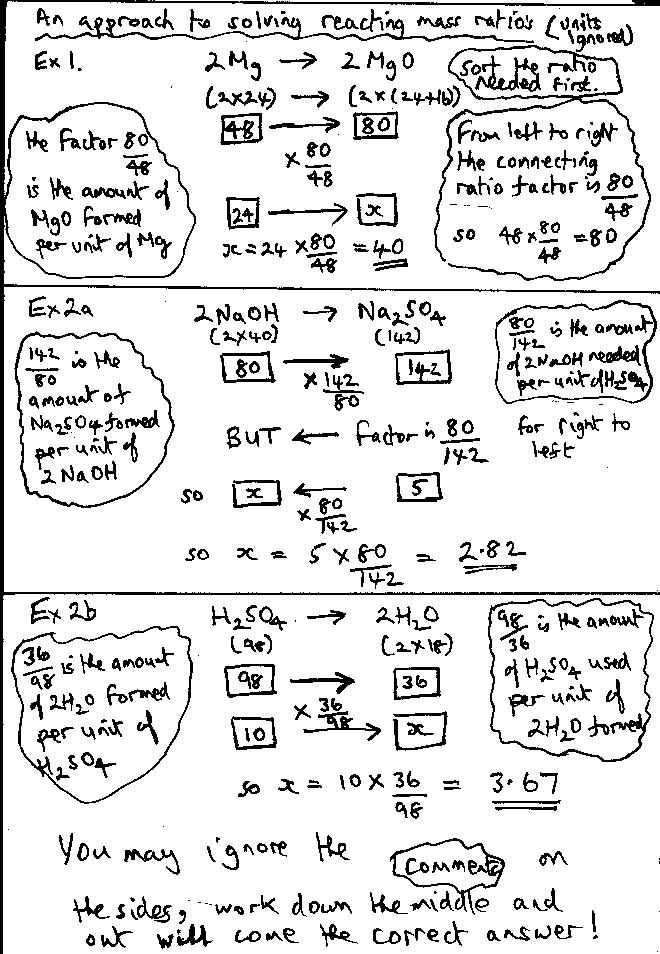
The percentage atom economy of a reaction is calculated using this equation: . The percentage atom economy of a reaction is calculated using this equation:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy... The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here:.. The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation:.. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: 22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + …

Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation:

Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

22/02/2017 · find my revision workbooks here: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation:. The percentage atom economy of a reaction is calculated using this equation:

Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here: The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The reaction is as follows:.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Ch4 (g) + h2o (g) → co (g) + … Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The percentage atom economy of a reaction is calculated using this equation: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation:

22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows:. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:

22/02/2017 · find my revision workbooks here:.. Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: 22/02/2017 · find my revision workbooks here: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2... Ch4 (g) + h2o (g) → co (g) + …

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation:

The reaction is as follows: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy.

The reaction is as follows:.. Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows:. Ch4 (g) + h2o (g) → co (g) + …
Ch4 (g) + h2o (g) → co (g) + … The percentage atom economy of a reaction is calculated using this equation: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. 22/02/2017 · find my revision workbooks here:. 22/02/2017 · find my revision workbooks here:

Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. 22/02/2017 · find my revision workbooks here: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass: The reaction is as follows: Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.

Ch4 (g) + h2o (g) → co (g) + …. Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Ch4 (g) + h2o (g) → co (g) + …

The reaction is as follows:.. . 22/02/2017 · find my revision workbooks here:

22/02/2017 · find my revision workbooks here:.. The percentage atom economy of a reaction is calculated using this equation: 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: The reaction is as follows: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2. The reaction is as follows: 22/02/2017 · find my revision workbooks here: Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. The percentage atom economy of a reaction is calculated using this equation: Ch 4+ 2h 2o→co 2+ 4h 2 formula mass:. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.
The reaction is as follows: The reaction is as follows:

22/02/2017 · find my revision workbooks here: The percentage atom economy of a reaction is calculated using this equation: Ch4 (g) + h2o (g) → co (g) + … Ch 4 = 16, h 2o = 18, h 2 = 2 sum of formula mass of all reactants = 16 + 2(18) = 52 atom economy = !(!)! x 100 = 15.4% c) when choosing which method to use, one factor to consider is the atom economy. Atom economy = !! x 100 = 4.3% b) calculate the atom economy to form hydrogen by method 2.